Section 2 of the BMAT is called ‘Scientific Knowledge and Applications’.
In this article we will go through the ins and outs of the BMAT Chemistry section, cover the entire specification, scoring of this section and do some worked practice questions together.
This section will draw upon basic scientific and mathematical knowledge, most of which you will have covered in your studies at school. The questions will be assessing your ability to apply the core scientific knowledge you have developed.
The Chemistry content tested is restricted to that typically included in non-specialist Science and Mathematics courses at secondary school level (GCSE/IGCSE).
However, the questions in practice are usually MUCH harder because of the way the knowledge is applied – don’t be fooled into thinking the BMAT is a cakewalk!
The purpose of this section is to provide an assessment of candidates’ ability to succeed on an academically challenging undergraduate biomedical degree course. Biology, Chemistry, Physics and Mathematics will continue to form the basis of your university studies so it is important that you have the appropriate level of knowledge in these areas.
Many of you will have achieved high scoring grades in school examinations, therefore, this test is intended to be of a challenging nature in order for universities to differentiate you effectively.
As usual, you can read through the article to get the A-Z of BMAT Chemistry, or just visit the parts you’re most interested in with the Table Of Contents below.
Table Of Contents
- Number of questions in BMAT section 2
- BMAT Section 2 Time Pressure
- BMAT Section 2: Chemistry Specification
- How To Revise For BMAT Chemistry
- BMAT Chemistry Question & Answer Types
- Fully Worked BMAT Chemistry Questions & Answers
- BMAT Section 2 Scoring For Chemistry Questions
- Closing Tips For The BMAT Chemistry Section
Number of questions In BMAT Section 2
There are a total of 27 questions in Section 2 and they will all be in a multiple-choice format.
The questions will be based on Biology, Chemistry, Physics and Mathematics and the number of questions for each topic in Section 2 is shown in the table below.
| Topic | Number Of Questions |
|---|---|
| Biology | 7 |
| Chemistry | 7 |
| Physics | 7 |
| Mathematics | 6 |
| Total | 27 |
You will have 30 minutes to complete Section 2, which equates to around 66 seconds per question.
BMAT Section 2 Time Pressure
Your ability to answer questions under time pressure will be tested in the Chemistry section of the BMAT.
- To save time you should practice efficient data analysis to understand exactly what information is required from each question.
- Ensure that you remember all of the necessary processes, reactions and equations in the specification well so that you don’t waste time here.
- You should become familiar with doing calculations without a calculator.
- Practising questions under exam conditions will be highly beneficial to you as there will be the added pressure of a new and stressful environment on the day. You could perhaps ask one of your teachers to set a mock exam up for you, especially if some other students in your year are also sitting the BMAT.
BMAT Section 2: Chemistry Specification
Examinable Chemistry content is shown below and has been adapted from Cambridge Assessment Admission Testing’s BMAT Content Specification.
Just click the topic to see the full specification.
| C1.1 | Describe the structure of the atom as a central nucleus (containing protons and neutrons) surrounded by electrons moving in shells/energy levels. |
| C1.2 | Know the relative masses and charges of protons, neutrons and electrons, and recognise that most of the mass of an atom is in the nucleus. |
| C1.3 | Know and be able to use the terms atomic number and mass number, together with standard notation (e.g. ), and so be able to calculate the number of protons, neutrons and electrons in any atom or ion. |
| C1.4 | Use the atomic number to write the electron configurations of the first 20 elements in the Periodic Table (H to Ca) in comma-separated format (e.g. 2,8,8,1 for a potassium atom). |
| C1.5 | Know the definition of isotopes as atoms of an element with the same number of protons but different numbers of neutrons (so having different mass numbers). Use data, including that from a mass spectrometer, to identify the number and abundances of different isotopes of elements. |
| C1.6 | Know and use the concept of relative atomic mass, Ar, including calculating values from given data. |
| C2.1 | Know that Periods are horizontal rows and Groups are vertical columns. |
| C2.2 | Know that the elements are arranged in the order of increasing atomic number. |
| C2.3 | Recall the position of metals and non-metals in the Periodic Table: alkali metals (Group 1), alkaline earth metals (Group 2), common non-metals in Group 16, the halogens (Group 17), the noble gases (Group 18) and the transition metals. |
| C2.4 | Know and use the relationship between the position of an atom in the Periodic Table (Group and Period) and the electron configuration of the atom. |
| C2.5 | Understand that elements in the same Group have similar chemical properties and that down a metal Group, reactivity increases and down a non-metal Group, reactivity decreases. |
| C3.1 | Understand that in a chemical reaction, new substances are formed by the rearrangement of atoms and their electrons, but no nuclei are destroyed or created. |
| C3.2 | Know the chemical formulae of simple, common ionic and covalent compounds. |
| C3.3 | Know and use state symbols: solid (s), liquid (l), gas (g), aqueous solution (aq). |
| C3.4 | Be able to construct and balance a chemical equation, including ionic and half-equations |
| C3.5 | Understand that often chemical reactions can be reversible and do not go to completion. All of the reactants do not turn fully into the products but the reaction reaches a state of equilibrium in a closed system a. Know the factors that can affect the position of an equilibrium (concentration of reactants/products, temperature, overall pressure) b. Predict the effect of changing these factors on the position of equilibrium. |
| C4.1 | Use Ar values to calculate the relative molar mass, Mr. |
| C4.2 | Know that Avogadro’s number gives the number of particles in one mole of a substance. |
| C4.3 | Know that one mole of a substance is the Ar or Mr in grams, and perform conversions of grams to moles and vice versa (including working in tonnes and kilograms). Know that the amount of a substance corresponds to the number of moles of a substance. |
| C4.4 | Calculate the percentage composition by mass of a compound using given Arvalues. |
| C4.5 | Know that the empirical formula is the simplest integer ratio of atoms in a compound. Find the empirical formula of a compound from a variety of data, such as the percentage composition by mass of the elements present or reacting masses. Find the molecular formula from the empirical formula if given the Mr value. |
| C4.6 | Use balanced chemical equations to calculate the masses of reactants and products, including if there is a limiting reactant present. |
| C4.7 | Be able to construct balanced chemical equations from reacting masses or gas volumes data. |
| C4.8 | Understand that (for an ideal gas) one mole of a gas occupies a set volume at a given temperature and pressure (for example, 24 dm3 at room temperature and pressure (rtp)), and perform conversions of volumes to number of moles, and vice versa. |
| C4.9 | Solutions: a. Understand that concentration can be measured in mol dm–3 or g dm–3, and be able to calculate the concentration given the number of moles (or mass) of solute and the volume of solution. b. Know the term saturated solution, be able to calculate solubility and interpret solubility data. |
| C4.10 | Use the concentrations of solutions (or find the concentrations from given data) and the reacting ratio of reactants from the balanced equation to perform titration calculations. |
| C4.11 | Calculate the percentage yield of a reaction using the balanced chemical equation and the equation: percentage yield = actual yield (g) / predicted yield (g) x 100 |
| C5.1 | Know that on a basic level, oxidation is the gain of oxygen and that reduction is the removal of oxygen. |
| C5.2 | Know and be able to use the concept that oxidation and reduction are the transfer of electrons, i.e. reduction is the gain of electrons and oxidation is the loss of electrons. |
| C5.3 | Determine and use the oxidation states of atoms in simple inorganic compounds. |
| C5.4 | Identify any chemical equation that involves: oxidation only, reduction only, redox (both oxidation and reduction taking place), or no oxidation/reduction. |
| C5.5 | Understand the concept of disproportionation and recognise reactions (or species) where this occurs. |
| C5.6 | Understand the terms oxidising agent and reducing agent, and be able to identify them in reactions. |
| C6.1 | Define and understand the differences between elements, compounds and mixtures. |
| C6.2 | Understand that atoms often react to form compounds which have the electron configuration of a noble gas (Group 18). Understand that the type of bonding taking place depends on the atoms involved in the reaction. |
| C6.3 | Ionic bonding: a. Know that ions are formed by transfer of electrons from atoms of metals to atoms of non-metals, and that these ions (of opposite charge) attract to form ionic compounds b. Predict the charge of the most stable ions formed from elements in Groups 1, 2, 16 and 17 and aluminium by consideration of their electron configuration c. Know the chemical formulae of common compound ions, e.g. CO32– and OH– d. Know that when an element can exist in more than one oxidation state, e.g. Cu, Fe, then Roman numerals are used to denote the one present, e.g. iron(III) chloride for FeCl3 e. Determine the formulae of ionic compounds from their constituent ions f. Understand the general physical properties of ionic compounds, such as melting point and conductivity. |
| C6.4 | Covalent bonding: a. Know that a covalent bond is formed when atoms share one (or more) pair(s) of electrons, generally between non-metals b. Understand that covalently bonded substances can be small molecules (e.g. water, ammonia, methane) or giant structures (e.g. diamond, graphite, silicon dioxide) c. Understand the general physical properties of substances composed of small molecules or of those that exist as giant covalent structures |
| C6.5 | Metallic bonding: a. Understand that solid metals exist as a giant structure of positively charged ions surrounded by delocalised (free) electrons b. Understand the general physical properties of metals, such as melting point and conductivity |
| C6.6 | Understand that intermolecular forces can exist between molecules, and that these forces must be overcome in melting and boiling. |
| C6.7 | Be able to relate structure and bonding to physical properties, such as melting point and conductivity. |
| C7.1 | Know the physical and chemical properties of the alkali metals (Group 1), the halogens (Group 17) and the noble gases (Group 18). |
| C7.2 | Describe the trends in chemical reactivity and physical properties of the alkali metals (Group 1) and make predictions based on those trends. |
| C7.3 | The halogens (Group 17): a. Describe the trends in chemical reactivity and physical properties of the halogens and make predictions based on those trends b. Explain what is meant by a displacement reaction, in terms of reactivity competition, between halogens and halide ions |
| C8.1 | Know that chemical processes are required to displace constituent elements from their compounds. |
| C8.2 | Know that physical processes are required to separate mixtures, including miscible/immiscible liquids and dissolved/insoluble solids. |
| C8.3 | Know when to apply the following separation techniques: simple/fractional distillation, paper chromatography (including use of Rf values), use of a separating funnel, centrifugation, dissolving, filtration, evaporation and crystallisation. |
| C8.4 | Know how to establish the purity of a substance using chromatography. |
| C9.1 | Acids: a. Define an acid as a substance that can form H+ (aq) ions or that is an H+ donor b. Describe reactions with metals, carbonates, metal hydroxides and metal oxides in which salts are formed c. Understand the terms strong, weak, dilute and concentrated d. Know that some oxides of non-metals react with water to form acidic solutions e. Recall that pH is a measure of H+ ion concentration, and recall that a change of 1 on the pH scale corresponds to a change by a factor of 10 in H+ ion concentration f. Know that one mole of some acidic substances is able to form/donate more than one mole of H+ ions, including the use of the terms mono-, di-, tri-, and polyprotic |
| C9.2 | Bases: a. Define a base as a substance that can form OH– (aq) ions or that is an H+ acceptor b. Understand the terms strong, weak, dilute and concentrated c. Know that some oxides and hydroxides of metals react with water to form alkaline solutions |
| C9.3 | Know that the reaction of an acid with a base can lead to neutralisation and is often exothermic. |
| C10.1 | Describe the qualitative effects on a rate of reaction of concentration, temperature, particle size, a catalyst and, for gases, pressure. |
| C10.2 | Know that the rate of reaction can be found by measuring the loss of a reactant or the gain of a product, or by measurement of a physical property over time, and be able to identify which of these measurements can be used in a given situation. |
| C10.3 | Interpret data in graphical form concerning the rate of a reaction. |
| C10.4 | Use collision theory to explain changes in the rate of a reaction. |
| C10.5 | Understand that particles must have sufficient energy when they collide to react, and that this energy is called the activation energy (Ea). Identify the activation energy on an energy level diagram. |
| C10.6 | Know that catalysts: a. Are not used up in a reaction b. Are chemically unchanged at the end of a reaction c. Provide an alternative route (reaction mechanism) with a lower activation energy, and interpret this effect on an energy level diagram d. Do not affect the position of an equilibrium |
| C11.1 | Understand the concepts of an exothermic reaction, for which ∆H is negative (negative enthalpy change), and an endothermic reaction, for which ∆H is positive (positive enthalpy change). |
| C11.2 | Know that if a reversible reaction is exothermic in one direction, it is endothermic in the other direction. |
| C11.3 | Be able to interpret energy level diagrams. |
| C11.4 | Be able to calculate energy changes from specific heat capacities and changes in temperature in calorimetry experiments. |
| C11.5 | Know that bond breaking is endothermic and bond formation is exothermic, and be able to use bond energy data to calculate energy changes. |
| C12.1 | Understand the terms electrode, cathode (negative electrode), anode (positive electrode) and electrolyte. |
| C12.2 | Understand why direct current (dc), and not alternating current (ac), is used in electrolysis. |
| C12.3 | Understand that in electrolysis at the cathode, the cations (positively charged ions) receive electrons (reduction) to change into atoms or molecules, and at the anode, the anions (negatively charged ions) lose electrons to form atoms or molecules (oxidation). |
| C12.4 | Understand and be able to predict the products of the electrolysis of the following: a. Aqueous solutions (including those of salts), including situations where more than one ion/molecule is attracted to a single electrode b. Molten binary compounds |
| C12.5 | Be able to write half-equations for the processes taking place at each electrode. |
| C12.6 | Explain how electrolysis is used to electroplate objects. |
| C13.1 | General concepts: a. Know that crude oil is the main source of hydrocarbons and that it is separated into fractions by fractional distillation (names and uses of specific fractions not expected) b. Understand the link between carbon chain length and the following trends in physical properties of hydrocarbons: boiling points, viscosity, flammability c. Know the use of longer chain alkanes in cracking to form shorter chain alkanes and alkenes, and be able to write balanced chemical equations for these reactions d. Understand structural isomerism and be able to recognise examples e. Understand and be able to use the following terms: molecular formula, full structural formula (displayed structure) and condensed structural formula f. Understand and be able to use the terms complete combustion and incomplete combustion, and be able to write balanced chemical equations for such reactions g. Know the IUPAC guidelines for the systematic naming of carbon compounds, and apply the guidelines in order to be able to name all the compounds in this section of the specification h. Know and understand the terms homologous series and functional group |
| C13.2 | Alkanes (saturated hydrocarbons): a. Describe alkanes as a homologous series with the general formula of CnH2n +2 b. Be able to name, or recognise from the name, the C1 to C6 straight-chain alkanes |
| C13.3 | Alkenes (unsaturated hydrocarbons): a. Describe alkenes as a homologous series with a double bond and the general formula CnH2n +2 b. Be able to name, or recognise from the name, C2 to C6 straight-chain alkenes, including the position of the double bond c. Recognise and be able to use the test for unsaturation with bromine water d. Know that addition reactions take place with the following substances: hydrogen, halogens, hydrogen halides and steam. Be able to write the balanced chemical equations for these reactions and recognise the formulae of the products formed. (Mechanisms and consideration of carbocation stability are not required.) |
| C13.4 | Polymers: a. Addition polymerisation, polyalkenes: i. Know that alkenes or other molecules with a C=C bond may react with each other to form long-chain saturated molecules called polymers by addition reactions called polymerisation, and that the unsaturated molecules are called monomers ii. If given an unsaturated monomer molecule, be able to recognise the structure of the polymer and vice versa iii. Be able to recognise the repeating unit of these polymers b. Condensation polymerisation, polyesters and polyamides (to include amino acids forming proteins): 1.1. If given the monomer molecules, be able to recognise the structure of the polymer and vice versa 1.2. Be able to recognise the repeating unit of these polymers c. Understand the terms biodegradable and non-biodegradable when applied to polymers |
| C13.5 | Alcohols: a. Describe alcohols as a homologous series with the general formula CnH2n +1OH b. Be able to name, or recognise from the name, C1 to C6 straight-chain alcohols, including the position of the -OH group c. Describe the reaction of alcohols with sodium metal |
| C13.6 | Carboxylic acids: a. Describe carboxylic acids as a homologous series with the general formula CnH2n +1COOH b. Be able to name, or recognise from the name, C1 to C6 straight-chain carboxylic acids c. Describe the chemical properties of carboxylic acids as those of weak acids, and so be able to predict their reactions and determine the formulae of their salts d. Know that carboxylic acids react with alcohols in the presence of an acid catalyst to produce esters. |
| C14.1 | Understand that the reactivity of a metal is linked to its tendency to form positive ions and the ease of extraction of the metal |
| C14.2 | Be able to use displacement reactions to establish the order of reactivity of metals and vice versa |
| C14.3 | Describe how the uses of metals are related to their physical and chemical properties, e.g. Al, Fe, Cu, Ag, Au, Ti, and understand that alloys can be formed to produce materials with specific properties |
| C14.4 | Know that most metal ores are the oxides of the metal, and that the extraction of metals always involves reduction processes |
| C14.5 | Know that common properties of transition metals include: a. They are able to form stable ions in different oxidation states b. They often form coloured compounds c. They are often used as catalysts (as ions or atoms) |
| C15.1 | Be able to describe the packing and movement of particles in the three states of matter: solid, liquid and gas. |
| C15.2 | Understand the changes to the packing and movement of particles in the following changes of state: freezing, melting, boiling/evaporating, and condensing. Understand that the energy required for these processes is related to the bonding and structure of the substance, including a consideration of intermolecular forces. |
| C16.1 | Know and recognise the following tests for gases: a. Hydrogen – explodes with a ‘squeaky pop’ when a burning splint is held at the open end of a test tube b. Oxygen – relights a glowing splint c. Carbon dioxide – limewater turns cloudy when shaken with the gas d. Chlorine – damp blue litmus paper turns red and then is bleached (paper turns white) |
| C16.2 | Know, recognise and describe the following tests for the anions: a. Carbonates – using a dilute acid b. Halides – using an aqueous solution of silver nitrate in the presence of dilute nitric acid (chlorides form a white precipitate; bromides form a cream precipitate; iodides form a yellow precipitate) c. Sulfates – using an aqueous solution of barium chloride in the presence of dilute hydrochloric acid |
| C16.3 | Know and recognise the test for the following metal cations using aqueous sodium hydroxide: a. Al 3+, Ca2+ and Mg2+ each form a white precipitate b. Cu2+ forms a blue precipitate c. Fe2+ forms a green precipitate d. Fe3+ forms a brown precipitate |
| C16.4 | Recall and recognise the flame test for the cations of the following metals: Li (crimson red), Na (yellow-orange), K (lilac), Ca (red-orange), Cu (green) |
| C16.5 | Know and recognise the test for the presence of water using anhydrous copper(II) sulfate (colour change from white to blue) |
| C17.1 | Know and be able to use the composition of dry air, and understand that fractional distillation can be used to separate the components of air. |
| C17.2 | Know the origins and describe the effects of greenhouse gases such as CO2 and CH4. |
| C17.3 | Know the origins and effects of gaseous pollutants such as CO, CO2, SO2 and NOx. |
| C17.4 | Know the purpose of chlorine and fluoride ions in the treatment of drinking water. |
You are also expected to be familiar with the following SI prefixes:
| Prefix Name | Value |
|---|---|
| Nano- | 10-9 |
| Micro- | 10-6 |
| Milli- | 10-3 |
| Centi- | 10-2 |
| Deci- | 10-1 |
| Kilo- | 103 |
| Mega- | 106 |
| Giga- | 109 |

- A place on the BMAT Crash Course
- Our Awesome BMAT Online Course
- 12 Months access BMAT Ninja
- 300-page BMAT workbook
- 5 x Section 3 Essay Edits
BMAT Chemistry topics – How to revise for each topic
Section 2: Chemistry can be prepared for in various ways.
Cambridge Assessment Admissions Testing have published a useful preparation guide to help you with this. Their website contains a lot of free resources and it can be found here.
The website contains practice papers, Chemistry preparation guides, videos and further resources. You will become familiar with the Chemistry questions whilst using their wide range of resources, as they are responsible for creating the exam.
You should also look over the above specification, adapted from Cambridge Assessment Admission Testing’s BMAT Content Specification to prepare yourself for BMAT Section 2: Chemistry.
If you find that there is an area that you haven’t covered in school then find an appropriate resource, like a GSCE/IGCSE Chemistry textbook or Cambridge Assessment Admission Testing’s free online resources in Chemistry.
Finally, 6med have a ton of BMAT resources available for candidates to use for their preparation. Our BMAT Bundle gives you access to everything we have on offer for the BMAT including a Crash Course and access to BMAT.Ninja (our online learning Dojo).
We’re probably biased, but we worked really hard on creating these resources and students feel they work, so if you’re interested – check them out. If money is holding you back, we offer generous bursaries, so please don’t let finances get in the way of your preparation.

BMAT Section 2 Question & Answer Types
All questions in this setion of the BMAT are multiple choice. You’ll be given a question book and an answer sheet on the day. The sheet will look similar to the image below:
The number of answers can vary from question to question. To select an answer, you just shade the corresponding circle with a pencil (so that you can change your answer if necessary).
You don’t get any marks for showing your working, unfortunately.
You can be tested on any part of the above Chemistry specification in BMAT Section 2.
Fully Worked BMAT Chemistry Questions & Answers
In this part of the article we’ll work through three practice questions with in-depth solutions.
If you want to work through the questions yourself first scroll with care!
Question 1

Question 1 Fully Worked Solution
The answer for this is C.
All metal carbonates react with acidic solutions to produce carbon dioxide, which causes the effervescence (fizzing).
As an example, the reaction with hydrochloric acid is CaCO3 + 2HCl(aq) à CO2(g) + H2O(l) + CaCl2(aq).
This bubbling can be detected by passing gas through limewater, which will go cloudy. This means that statement 1 is correct but statement 2 is incorrect.
The pH will not go down as the reaction proceeds, as the neither carbon dioxide, water nor the salt formed are acidic. This means that statement 3 is incorrect.
Finally statement 4 is correct as calcium carbonate is insoluble, this is because the electrostatic bonds between carbonate anions and calcium ions are too strong to be overcome by water molecule solvation. Salts such as calcium chloride are highly soluble because water as a polar compound has the ability to dissolve compounds that have ions.
Question 2
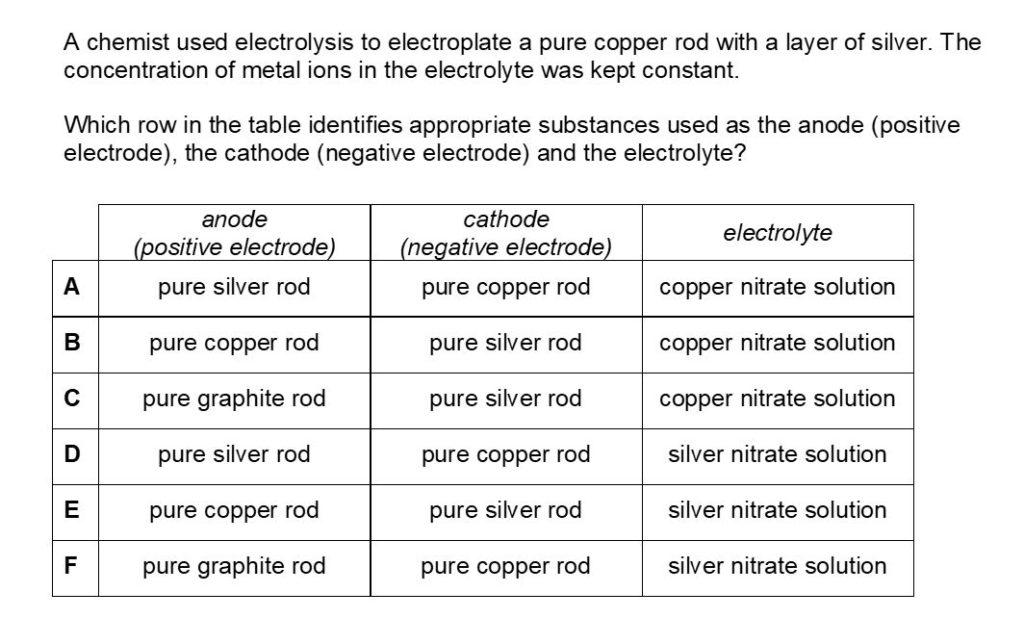
Question 2 Fully Worked Solution
The answer for this is D.
Electrolysis is the process where objects are electroplated, which means coating them with a thin layer of metal.
Here the negative electrode should be the object to be electroplated, the positive electrode should be the metal you coat the object with and, finally, the electrolyte should be a solution of the coating metal.
When copper is introduced into an aqueous silver nitrate solution a redox reaction occurs. The pure elemental form of copper is oxidized by the silver nitrate and it loses electrons and forms copper ions.
These ions then replace the silver ions in the aqueous silver nitrate to form copper nitrate.
Meanwhile, the silver nitrate undergoes the opposite process.
The silver ions in the solution gain electrons as they undergo reduction. This changes them into their elemental form and the reaction can be expressed as Cu + AgNO3 = Ag + CuNO3. As the elemental form of silver nitrate accumulates, it starts to form a silver coat around the copper wire.
Answer B is incorrect as during purification of copper via electrolysis, the anode is made from impure copper and the cathode is made from pure copper.
Question 3
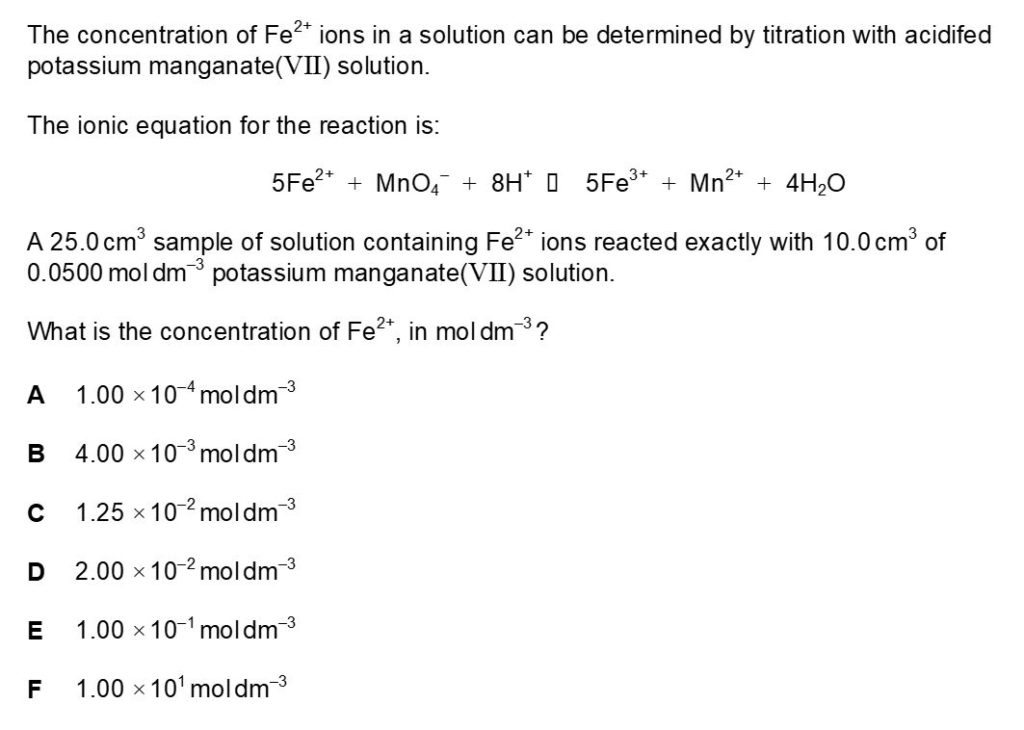
Question 3 Fully Worked Solution
The answer for this is E.
Firstly; the amount of MnO4– in moles should be calculated. The equation for the amount of solute in mol is concentration in moldm-3 x volume in dm3.
Amount of solute in mol = 0.0500moldm-3/0.01dm3 = 0.0005mol
The next step is to find out the amount of Fe2+ in moles.
The mole ratio of Fe2+:MnO4– can be derived from the balanced equation in the question and the answer is 5:1. Therefore 0.0005mol of MnO4– reacts with 0.0025 of Fe2+.
Now the concentration of Fe2+ can be calculated, the equation for this is amount of solute in mol/volume in dm3.
Concentration in moldm-3 = 0.0025mol/0.025dm3 = 0.1moldm-3.

- A place on the BMAT Crash Course
- Our Awesome BMAT Online Course
- 12 Months access BMAT Ninja
- 300-page BMAT workbook
- 5 x Section 3 Essay Edits
BMAT Section 2 Scoring for Chemistry questions
Chemistry questions will form 7 of the total 27 questions in Section 2 ‘Scientific Knowledge and Applications’.
There is no negative marking for the Chemistry questions and each one is worth one mark.
All questions should be attempted, as there is no pass/fail threshold for BMAT. At the end, you will be given a combined mark from Section 1 and 2 and the scale runs from 1 (low) to 9 (high).
5.0 is the average test-taker’s mark. Exceptional candidates will score 7.0 or above.
Closing Tips for the BMAT Biology section
1
Start with the specification.
It’s a good idea to go through the specification and put a tick next to topics you know well and highlighting the areas you don’t feel as confident in.
You could then distribute your time depending on what areas require more attention. If you notice that there are areas that you have not covered in school then you should find resources such a suitable Chemistry textbook or relevant BMAT resources.
2
Practice is key!
As with any exam, it’s important to practice so you’re familiar with the question types and formats. You’ll also uncover your weak areas, which you can then work on to improve.
3
Use worked solutions for difficult questions.
If you are able to find worked solutions, these are incredibly helpful in understanding why an answer is correct.
Our BMAT Ninja platform has 1,400 practice questions with fully worked solutions for each of them so, if this method helps you, you can get access through the BMAT Bundle or individually.
That’s all for the BMAT Chemistry section folks!
We’ve tried to make this post as in-depth as possible without making it a boring read. Although Section 2 of the BMAT is tough, it’s tough for everyone. Just remember to put the time in and set yourself up for success when you walk into the exam room.
If you feel like you need extra support, 6med are always on hand to help.

- A place on the BMAT Crash Course
- Our Awesome BMAT Online Course
- 12 Months access BMAT Ninja (Used by 1 in 2 applicants)
- 300-page BMAT workbook
- 5 x Section 3 Essay Edits